The global personal care ingredients market is worth approximately $12.85 billion, with over 16,000 chemicals registered for use in the personal care and beauty industry. Yet pre-20th century, for most people personal care was limited to a bar of soap and nothing more. So how did this industry boom from bar soap to billion-dollar business? Let’s take a look at the chemistry, history and trends of personal care.
The background to the bubbles
Soap is made by reacting triglycerides (natural fats) with alkali hydroxides (usually sodium) to produce salts of fatty acids. These molecules are made up of both water-soluble and oil-soluble groups, making them surfactants (more on that later), and perfect for cleaning.
The first recorded soap was created by the Babylonians from fats boiled with ashes around 2800 BC. From then on, soap making developed concurrently around the globe using locally available ingredients. Plant ashes and animal fats were most commonly used, but olive oil was also popular to produce a softer soap, and aromatic plants could be added to provide scent – introducing the importance of sensory properties in personal care.

Production remained small scale until the mid- to late 19th century, when the industrial revolution meant bar soap could be mass-produced. Paired with the advent of universal indoor plumbing, the doors were opened to the development of more sophisticated personal care products. In 1900, the first “water-in-oil” emulsion was patented (hello moisturizer!); the 1920s saw the introduction of liquid shampoo, and the 70s and 80s welcomed cationic surfactants into conditioner formulations (perfect timing for those perms!). So, what goes into the bottles in our bathrooms today?
Key chemistries
The ingredients in personal care products such as shampoo, shower gel or hair conditioner are labelled according to the International Nomenclature of Cosmetic Ingredients (INCI). They’re also listed in order of concentration (main ingredient first), that’s why water goes by “Aqua” and is usually number one. There are over 16,000 INCI registered chemicals, but don’t let that scare you – most of them can be grouped into key categories. Here are four examples with interesting chemistries:
1. Surfactants
Surfactants are the workhorse of the personal care formulator, thanks to their unique structure. Part water soluble (hydrophilic), part water insoluble (hydrophobic) − see figure 1 − these “surface active agents” can decrease surface tension, the cohesive forces between the molecules in a liquid. This leads to a wide scope of functionality, including the magic trick of mixing oil and water!
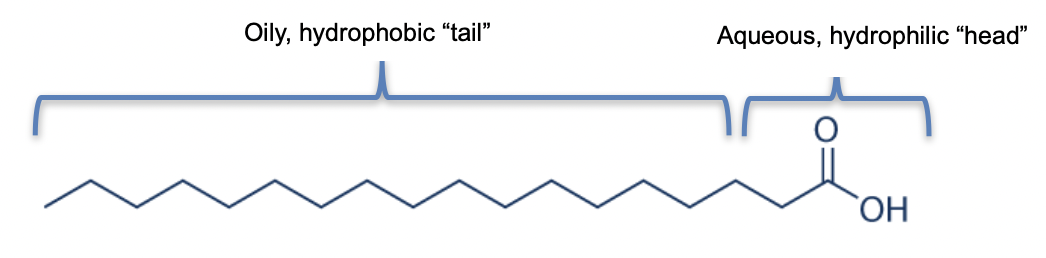
Figure 1: Example of a surfactant molecule, stearic acid: the original soap.
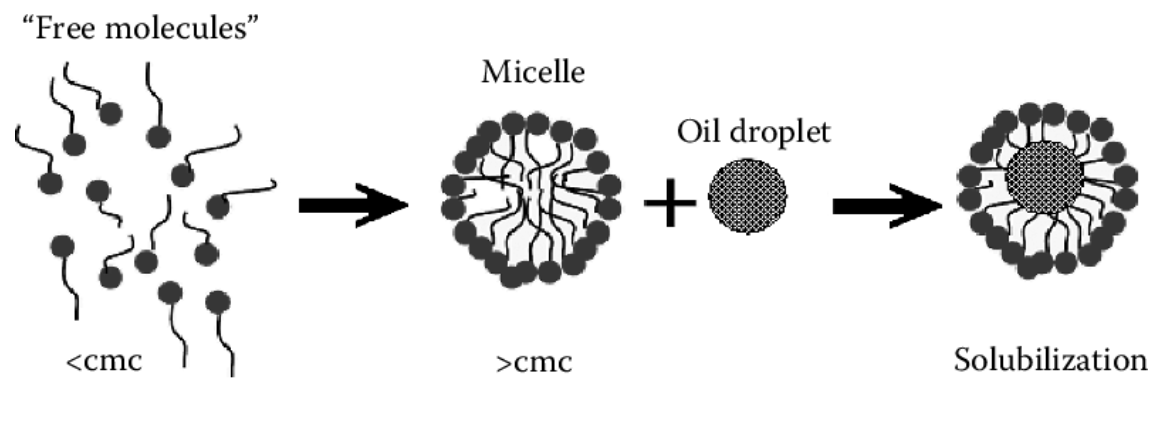
Surfactants are also the masters of self-assembly, spontaneously forming micelles in solution. Their oily tails congregate together to reduce the unfavourable oil-water surface interactions, resulting in a spherical structure. Why does this matter? Micelle formation enables surfactants to solubilise dirt as well as emulsify oil and water (see figure 2) – essential in many personal care products.
Foaming is another talent of surfactants. The attraction between the water molecules is weakened in the presence of surfactants, so the surface tension between air and water is lowered, allowing bubbles to form. Finally, some surfactants can act as conditioning agents. By adsorbing on to the surface of a hair cuticle via electrostatic attraction, they coat and smooth the hair, and making it easier to comb.
Surfactants are grouped according to their charge, which is key in determining their primary function in a personal care formulation − table 1 shows examples found in everyday products.
| Surfactant type | Famous for… | Found in… | You may recognise… |
| Anionic (negatively charged) |
Strong detergency and foaming | Shampoo, shower gel and even toothpaste | Sodium Lauryl Sulfate (SLS) Sodium Lauryl Ether Sulfate (SLES) |
| Cationic (positively charged) |
Conditioning | Hair conditioner | Benzalkonium Chloride Cetriumonium Chloride |
| Amphoteric (containing both positively and negatively charged groups) |
Gentle detergency | Sensitive skin products | Cocamidopropyl Betaine (CAPB) Disodium Cocoamphodiacetate |
| Nonionic (no charge) |
Emulsifying, detergency (esp. for oil/grease) | Shampoo, shower, gel, moisturiser… | Cocamide Monoethanolamine Fatty alclohol ethoxylates e.g. Laureth-3 |
2. Oils, fats and waxes
Oils, fats and waxes are the original moisturisers, helping to keep the skin or hair moist by providing a barrier that prevents water loss. Their physical properties can also help to thicken and structure a formulation, enhancing sensory properties for the consumer.
Natural oils are made up of fatty acids that have medium to long (6+ carbon atoms) hydrophobic hydrocarbon chains, see figure 3 for an example. It’s these chains that help coat the skin and lock in moisture. They also affect the “thickness” or viscosity of the solution – the longer the hydrocarbon chain, the higher the viscosity, or “thicker” the oil feels.
Commonly used natural oils include jojoba, avocado, olive, sunflower, soybean and coconut oil, which has even been shown to repair and protect damaged hair.
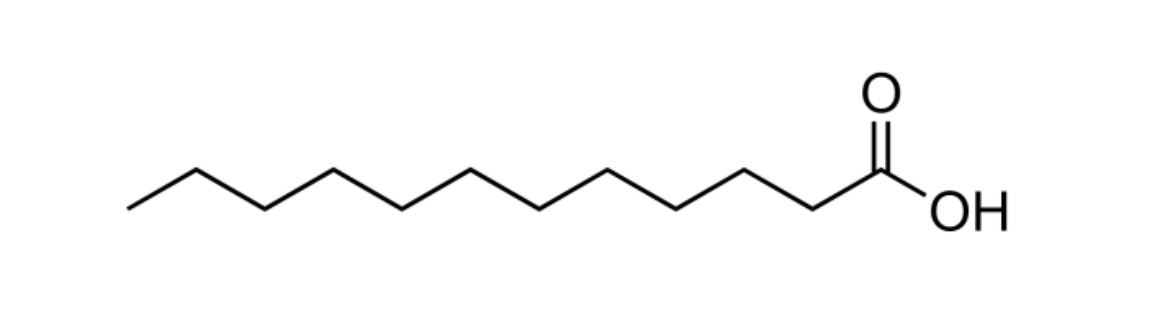
Figure 3: Lauric acid, a fatty acid with a chain length of 12 carbon atoms. Lauric acid makes up approximately half of the composition of coconut oil.
3. Polymers
Polymers are macromolecules – large molecules made up of repeating units, or monomers. They’re multifunctional, but their most common application in personal care is as thickening agents. Like oils, polymers can also act to increase the viscosity of a formulation. This not only enhances the “feel” of the product, but also improves spreadability and helps to ensure an optimum flow rate when you pour it from the bottle – rather than dripping straight from your hand to the shower floor!
Unlike oils, polymers don’t just rely on high molecular weight in thickening applications. Polyacrylates (polymers of acrylic acid) are commonly used in personal care formulations. They’re hydrophilic, and able to absorb as much as 100 to 1000 times their mass in water, to form a gel. The viscosity can be fine-tuned by varying the concentration and pH, making polyacrylates a really useful tool for formulators.
Polymers can also condition and moisturise. A common example used in hair conditioner is Dimethicone, a versatile hydrophobic silicon-based polymer that coats the hair to form a barrier against moisture loss, smoothing the hair’s surface and leaving a “shine”.
4. Chelates
Metal ions found naturally in water such as Magnesium (Mg2+) and Calcium (Ca2+) can catalyse the degradation of oils and fatty acids in personal care formulations, reducing shelf life. They can also make a product less effective, by reacting with surfactant molecules and reducing functionality.
Ever noticed a chalky build-up in your shower or kettle? You probably live in a hard water area. The “harder” the water is, the more metal ions are present, and the more product you’ll need to use to get the same level of detergency. That’s why you might find yourself getting through more shampoo in London (a hard water area) than in Manchester (a soft water area)!
The word chelate is derived from the Greek chela meaning crab claw. Adding chelating agents to a formulation sequesters the metal ions, binding them (like in a crab’s pincer) so they can no longer react with the ingredients. A common chelate is disodium EDTA. This molecule uses negative charges to bind the positively charged metal ions – see figure 4.
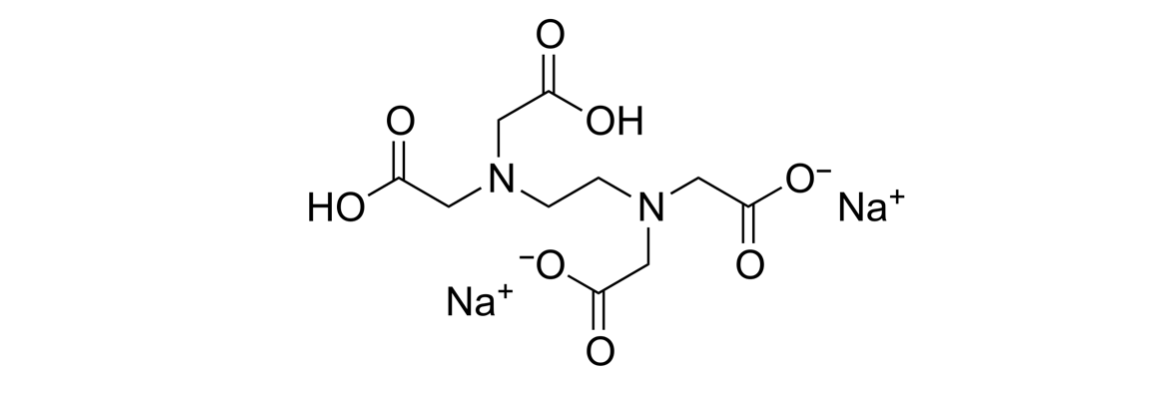
Figure 4. Disodium EDTA has negatively charged carboxylates and amine groups that can bind positively charged metal ions from solution.
Top trends and future focus
Despite all the chemistry behind it, personal care is very much a consumer led market influenced by wider global trends. During the pandemic, the industry saw an unprecedented increase in demand. With spa treatments, beauty salons and holidays off-menu, consumers chose to spend more on DIY self-care at home.
Today, there remains an increased focus on health and well-being, with more people setting aside time to take care of themselves. Products like face masks and luxury bath and skin care remain popular, using ingredients such as essential oils, argan oil and aloe vera. Premium active ingredients are also in demand, such as retinol for collagen building and hyaluronic acid for natural skin plumping.
The growing consumer awareness of climate change and demand for sustainability is leading to the development of plant-based ingredients such a bio-surfactants derived from sugar, as well as more transparency on how materials are sourced, and innovative packaging solutions like solid shampoo bars and refill options.
However, whether commodity or premium, synthetic or bio-based, the fundamental chemistries of personal care remain the same.
And next time you pick up a bottle of shower gel, you can appreciate the myriad interactions and functionalities of the molecules inside.